Heating the Substance Again. Distillation Circulation Coobation
Leonardo AnfolsiThe distillation has something magical, we can’t figure out why a substance gets refined through heat and the passing in another container, or even circulating, which is rising and falling in the same glass balloon.
We just know it works: through this process the substances lose some of their toxicity, become more digestible or refined, more alcoholic, more penetrating or concentrated.
Moreover, through distillation or movement, we can literally change the nature of some particular substances. This happens in particular when we put them through a specific degree of heat, under vacuum, or eventually when, during distillation, we use special devices; or if we add other reagents able to cement the substance or catalyze the process, in some cases functioning as a fire themselves.
The mystery of the shaping fire involves everything, every phenomena, the living ones as well. So there are several fires in our world, that give shape to the embryo, flavor and texture to the wine, a form to the trees, to the leaves, in a continuous process that never begins and never ends.
We can see through this mystery and appreciate the knowledge that comes therein, but only if we are able to consider this world as a whole living entity, not just some/thing separate from us.
Antique instruments for the eternal art/science
Once we understand the articulated simplicity of antique instruments, we will also fully understand how the modern and contemporary ones work, what expect from them and how regulate, change or contradict the standard use of them.
Let’s examine the general issues related to the transition from a balloon to a receiver.
- We need to produce the passage of the liquid into its gaseous form, from the distiller balloon to the receiver.
- The substances are of a different nature, being oily, alcoholic, thick or thin, liquid or with more phlegm, therefore their passage through the instruments could be more or less easy.
- We rather need only a part of the substance, leaving the other in the balloon or in the receiver to be used differently, to be thrown or to be disposed of.
- We need or prefer an easier and faster passage of the distillate therefore cooling it up.
- Or we strive to obtain our distillation with the lowest heat possible.
- We require a sublimation in the distillation, that is the transition of the substance from liquid and vapour to solid and crystallised.
- When the substance to be distilled is solid or the form of its passage is vapour, or thinner still, air or gas and the latter should be channeled through.
- And then eventually pushed to solidify or crystallise through cold temperature.
- Then there is the variable of coobation and the consequent need to easy get to the caput, remove and grind it to receive again the distilled liquid.
Considering what is described above, and more besides, there is in the alchemical tradition a fair amount of labware and three different traditional directions of distillation as it is outlined below:
Three different Directions in Ancient Distillation

1) For ascenso (by elevation), In general It needs cooling...

2) For the side

3) For descenso (by descending). It has a guide or some glass shape that leads the vapours to drip into the rostrum. This kind of shape like a pocket is present also in the oldest heads made appositely for precise phials.
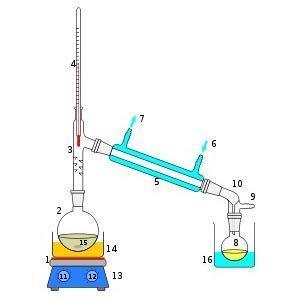
Parts of an Ancient system of Distillation

Diagram of the insertion phial head

- A - head, or helm[1]
- B - ursale (flask with large neck and opening) but here you could put also a vesica, a phial or a cucurbit
- C - chapel (large skillet = Arabic: Al Tannur i.e. athanor) with coarse sand (sand bath); it is often called "athanor" an blind alembic, a crucible, or a vase/egg (closed towards the end of certain works) or more often the whole of the coal furnace, a multifunctional tower that holds the plan for the covered crucible, down near the coal and higher up various chapels with flasks, et cetera
- D - rostrum, nose or beak possibly with coolant G
- A + D = alembic
- E - receptacle that receives = receiver
- F - source of heat: in ancient times it was coal of various origins, enclosed in a multifunctional tower, in the nineteen century it was coke coal "American" producing as little smoke as possible, then butane / propane and finally the electricity that, almost always, is the best form of heat: the safest form of heati and the easiest to adjust
- G - coolant
- K - normal neck of a bowl to insert more easily in a helm
- K1 - wide neck of ursale to retrieve more easily the kaput
- K2 - the longer neck of the so called "phials"
- K3 - very long neck to circulate better, or to force the vapours to rise more
The single parts of a distillation apparatus
In the past centuries clay, porcelain, stoneware, tin-plated copper and, in some cases, common glass were used: with the ever-present danger that a single drop of cold water on the hot piece of pottery or glass could crack them and stop the whole process.
The Lute. In fact there were a large amount formulas of lute, or lutum[2], substances used not only to close more precisely the joints, but also all those breaks and cracks inevitable in those days: even hardened paper was pasted on the stoneware, clay or glass ware, or strips of animal intestines tied on and then glued with ingenious mixtures.
The glass used in the laboratory were the same as the ones in the windows, but they were thicker, and blown with more expertise: then with the addition of boron and of silicon and, furthermore, with the fusion of the pieces in the mould, a new era in chemical glassware began, thanks to those products in boro-silicate that would have satisfied the dreams of the masters alchemists of the seventeenth century to have all, or almost all the laboratory instruments made of glass[3].

Frosted cones - Bowl or modern balloon
The Balloon. What once was known in Italian as boccia or bozza (bowl[4]) is now called pallone (eng. Balloon) and presents the same neck with a latest addition of a frosted cone and cap.
The urinale, the ursale, with its large neck, has this name for a good reason; it comes handy in the distillation of human dew and storm water, it helps to retrieve the final dregs. On the other hand these dregs might be collected when they are still damp, and left to dry on a Pyrex plate and then dried, on the same plate. The heat-resistant plate reminds us that they need to be slowly calcinated. In ancient times, when there was not a boro-silicate glass the zucker glas was used, that is, the glass used to build fruit bowls, very thick sugar bowls or exhibitors of candy.

The retort is an alembic in one piece that distills heavy substances, oily or difficult to pass through: it's a ball that bends and twists on itself producing a long beak that is more narrow towards the end, where the distillate is dripping.

The modern version and the original sketch of the flask designed by Dr. Erlenmeyer
The vesica is an oblong copper cylinder, often tinned, that facilitates the rising of the vapours and let them reach the helm without get restricted in the neck. The distillate descends due to refrigeration. Today the Erlenmeyer flasks are commonly used, and on these is placed a Liebig distiller; in the past a helm was placed on the tin coated copper vesica, that was coated so that no substances would suffer any change, e.g. a vitriolation, thus becoming poisonous.
The phial is very similar but made of another material, earthenware, stoneware or glass, and is also useful for aqueous distillates, vaporous, therefore airy, light. Good to distill fruit and spirits. Even this instrument could be replaced today by an Erlenmeyer flask and mounting a boron-silicate glass helm or, as already said, a Liebig distiller.
The cucurbit, as we mention before, has a long and wide neck and it gets attached to the helm.
In ancient times to let the substance circulate, and be processed ascendind and descending inside the flask, rather than distilled, they used the long neck flask, the matraccio, as it is still known today: its lower part is flatter but retains the shape of the neck as in the old times, very long and narrow completed with cone and frosted glass-stopper.
In the Anglo-Saxon world it is known as Florentine vase, florentine flask or a similar name, and in some variables as volumetric flask, in case it has a ground-glass stopper.

Volumetric flask with a ground-glass stopper - Florentine flask
To circulate, for the so-called rotations, or process the substance in one flask, today we use circulators, namely a set of several pieces of glassware joint together to allow the communication between the various parts, and also to slightly heat, expand and cool the substances.
For the same purpose, in the past, a retort and a container of the same shape and size were placed, well joined together and both heated; They were the so-called vasi di ritorno, return vessels; but also "blind" pieces were used with only one opening from which to fill and empty, already shaped for this purpose: they were the gemelli, the twins, two balloons joined together who have on the side of both helms, a beak descending (per descenso) in each other's body, and the “pelican”, or the “crane” a single balloon with a beak descending down inside itself.
Techniques and adjustments
We shall now put together the themes that will guide our choices.
We may find in whatever needs to be distilled more or less delicate principles that risk to be dispersed during the distillation as well as the fact already observed, that the distilled material, can rise more or less, depending on its weight.
It is important that the distillation apparatus can absorb air without losing vapour: if the apparatus was tightly closed, the distillation would be very slow and risky in case of flammable materials. Nowadays, with air tight frosted cones and plugs, it seems necessary to put a small opening for air where the apparatus is already in contact with the receiver; In the old times the problem was quite the opposite: to be able to close all the possible cracks in the apparatus.
There are indeed two systems used to deal with the weight of the substance: on one hand the distillation “per ascenso”, by letting the substance rise, on the other hand the many adjustments to the pieces we already have; if the retort, through which we can distill heavy or oily substances in the best way, is not available, the balloon or the rostrums can be tilted, distilling per descenso, by letting the distilled substance go down.
Considering the heat, the barometric pressure, and the lunation, we can see the quantity that gets distilled, the time needed and the quality of the distilled substance; sorry just after watching some distillations you will know what to do.
In the distillation per ascenso (by elevation), we can stack various glasses before reaching the helm and the rostrum, or a long neck cucurbit with distillation per descenso (by descent) or by the side we can tilt the balloon we are heating up, provided it is enough empty.
Compared to the distillation by the side, the distillation per descenso is for heavier substances; as it is the distillation by retort.


Distillation by the side: dry wood, bones, horns, blood, gums, resins and tears, honey and sugar, and salts like vitriol, alum, saltpetre, common salt.
Distillation per descenso: is useful for the oils and in this case the retort can be used as well. The per-descenso apparatus can be useful, in the urine process when you need to sublimate its salts in the beak/rostrum, for example, of a liebig distiller.
All the distillers apparatus can be refrigerated on the rostrum (Liebig condenser) some inches after the curve, since we need for the steam to easily overcome the curve; another way to cool, then be condensed; the distillation is with ice around the receiver. The best solution - specially in this case - would be to use an ice maker machine and then, to cool the part around the rostrum, a motor for aquarium that send the icy waters to circulate in the coolant around the rostrum. An easier way is using PVC iced bottles immersed in a bucket full of cold water; It would be better than wasting the tap water.
Beware. An important aspect of Alcohol distillation
When we distill alcohol we must consider that the first drops that come out are named “the head of distillation” and they must be removed. The head contains the toxic substances; they can be detected by the smell that seems of chemical solvents. Indeed acetone starts to evaporate as soon as the distilling alcohol reaches 56 °C, the methanol at about 65 °C, and we must get rid of them at every distillation. In the next distillation, if you go on coobate, it is good to let few drops pass through and throw them, they can be recognised by their offensive smell. Nobody tells that; I wander about the quality of the ancient spirits…

Sketch taken from a notebook by Leonardo da Vinci

Codex Atlanticus, folio 216 r - Improved Alembic, study by Leonardo da Vinci

Keck clips for laboratory glassware
1. Sometimes imprecisely defined alambicco - alembic that actually means the whole device.
2. This is the ”lute of knowledge”, as explained by Isaac Newton in his notebooks: lutum sapientiae cum mastice preparandum. Raymund in Th. Ch. v. 4 p. 181. cum gypso et albugine ovi vel farina et albumine ovi Arnold in Th. Ch. v. 4. p. 550. et in Arte Aurif. part. 3. p. 160. Ex flore farinae albuminibus ovorum distemperato. Raymund in Th. Ch. v. 4. p. 141. Ex cera Anonym. in v. 3 Th. Ch. p. 13. cum olibano vel mastice molli vel viva calce et Ovorum albumine. Raymund. de quintessentia p. 25. cum bolo armeni, calce viva claro ovorum et pulvere vitri aequaliter mixtis Plinius Philos in Th. Ch. vol 6 p. 478. Morien. Rom. p. 33. University of Indiana biblioteque
3. The common glass is made by quartz sand, a fluxing agent to lower the melting point of the crystal and a stabilizer: the flux is soda (Na2CO3) or potash (K2CO3), the stabilizer is lime (CaCO3). Please notice that here there are at least two important alchemic ingredients, that are quartz sand (flint) and lime.
4. Again: bowl here means not a cup but a pot.